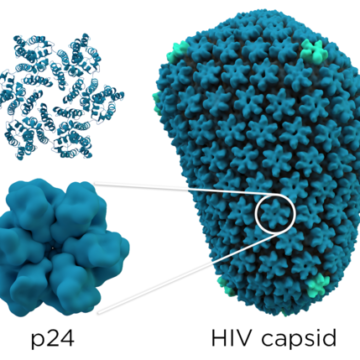
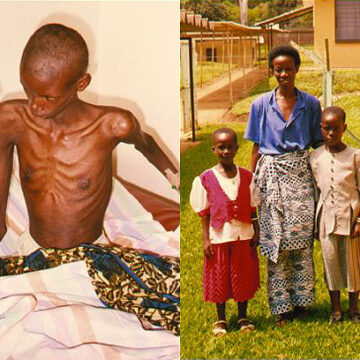
The daily use of a pill such as Truvada has been a mainstay of the fight against HIV infections for over a decade. This kind of preventive medication, called pre-exposure prophylaxis, or PrEP, has demonstrated impressive efficacy in clinical trials, with up to 99% success rate in preventing new HIV infections that are contracted through...
Tag: Lenacapavir
Home
Lenacapavir
Post
06/06/202406/06/2024HEALTH
New NIH Trials Aim to Address HIV Inequities in Women and Intravenous Drug Users
On Tuesday, the National Institutes of Health (NIH) declared the commencement of two noteworthy clinical trials that are intended to assess the efficacy and safety of an HIV pre-exposure prophylaxis (PrEP) medication among marginalized demographic groups. This effort, which focuses on groups like women and intravenous drug users that have previously been underrepresented in clinical...