Adults suffering from acute ischemic stroke are advised to consider tenecteplase as a therapy option by the National Institute for Health and Care Excellence (NICE). This choice represents a major advancement in the treatment of strokes and may have a big effect on patient care and NHS budget.
Boehringer Ingelheim’s Tenecteplase, also marketed under the trade name Metalyse, has shown clinical efficacy on par with alteplase, a more costly drug that NICE also suggests for the same indication. Following an acute ischemic stroke, both medications function by dissolving current blood clots and inhibiting the formation of new ones. NICE has indicated that the NHS might save millions of pounds by implementing tenecteplase because it is less expensive than alteplase.
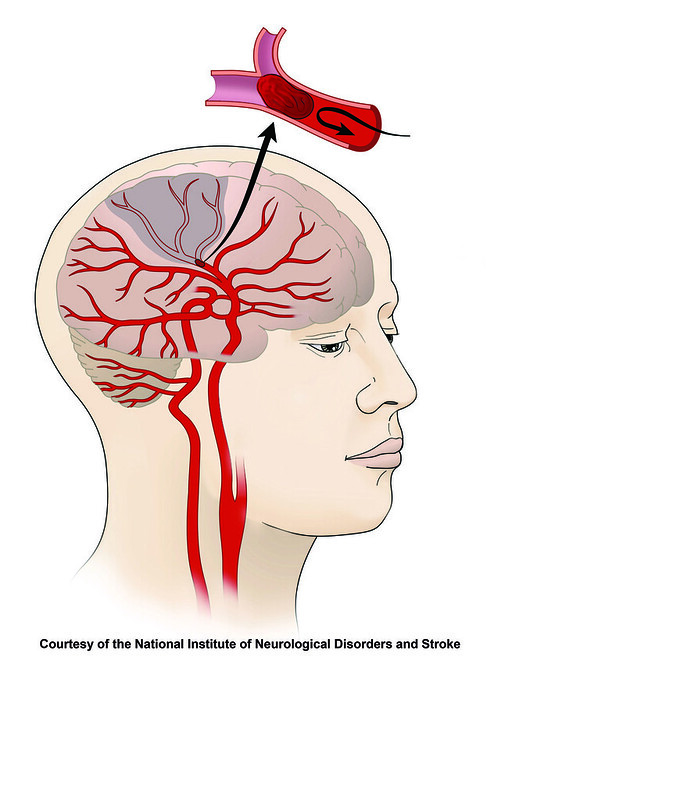
Tenecteplase must be administered in the early stages of a stroke, preferably within 4.5 hours of the onset of symptoms, and only after cerebral hemorrhage has been ruled out. By stimulating the synthesis of plasmin, an enzyme that dissolves blood clots, this drug aids in reestablishing blood flow via the damaged artery.
Tenecteplase was first licensed in the UK in April 2024 to treat ischemic stroke after it was first approved for the treatment of acute myocardial infarction (MI). NICE’s director of medicines evaluation, Helen Knight, stressed the value of prompt treatment for stroke victims, pointing out that the condition is still one of the main causes of mortality and disability. Knight emphasized that the new guidelines help the NHS save money while simultaneously giving patients another alternative for treatment.
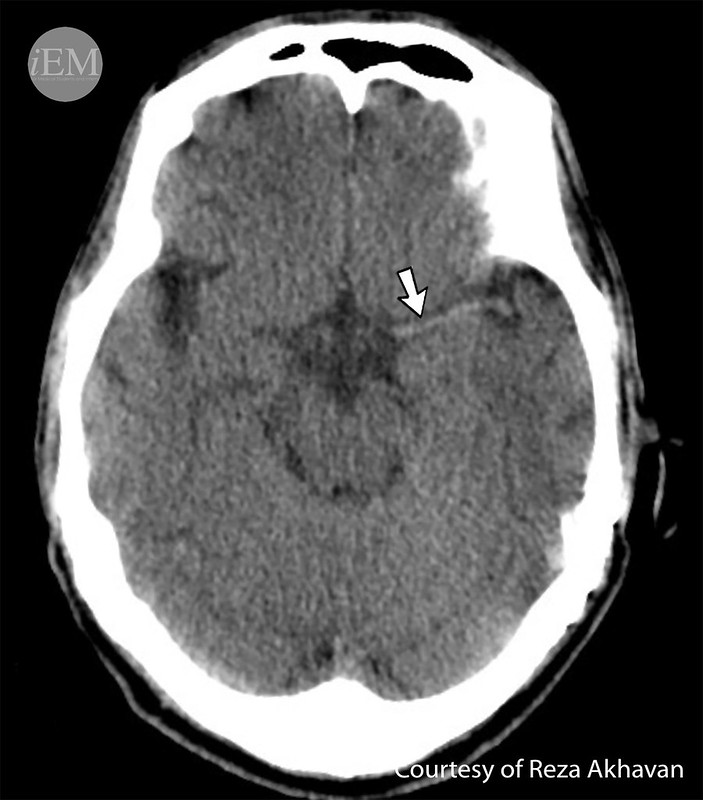
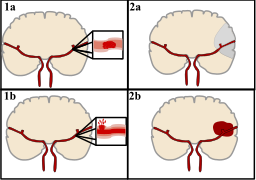
About 85% of all stroke occurrences are ischemic strokes, which happen when a blood clot blocks a portion of the brain’s regular blood flow. This obstruction causes oxygen deprivation in the brain, which damages or kills cells and causes symptoms similar to a stroke. An estimated 100,000 persons in England are admitted to hospitals each year as a result of stroke, the bulk of which are ischemic strokes. In this nation, more than a million people deal with the chronic consequences of stroke.
Professor of clinical imaging and consultant neurologist Keith Muir of the University of Glasgow offered commentary on NICE’s ruling, emphasizing the opportunity for major cost and patient care reductions. He highlighted the useful benefits of tenecteplase, including its quick and simple administration. Tenecteplase is given as a simple single-dose injection, in contrast to alteplase, which needs a complicated administration procedure consisting of an initial bolus followed by an intravenous infusion spread out over an hour. Because of its simplicity, patients can receive prompt, effective care without having to worry about the practicalities of continuing infusion—even if they must be transferred between institutions.
Muir cited research from New Zealand that was published in July 2023 and indicated that patients treated with tenecteplase had higher chances of success than those treated with alteplase. He underlined that treatment must begin quickly in order for recovery to occur; the sooner intervention begins, the more likely it is that recovery will be favorable. Thus, tenecteplase has obvious and significant practical benefits in real-world situations.
Boehringer Ingelheim experienced manufacturing difficulties in 2022 that impacted the world’s supply of tenecteplase and alteplase, prompting hospital trusts to ration their stock. Despite these difficulties, NICE’s recommendation for tenecteplase marks a substantial improvement in the management of acute ischemic stroke and presents a viable solution for enhancing patient outcomes while also addressing cost-effectiveness within the National Health Service (NHS).
Leave a Reply